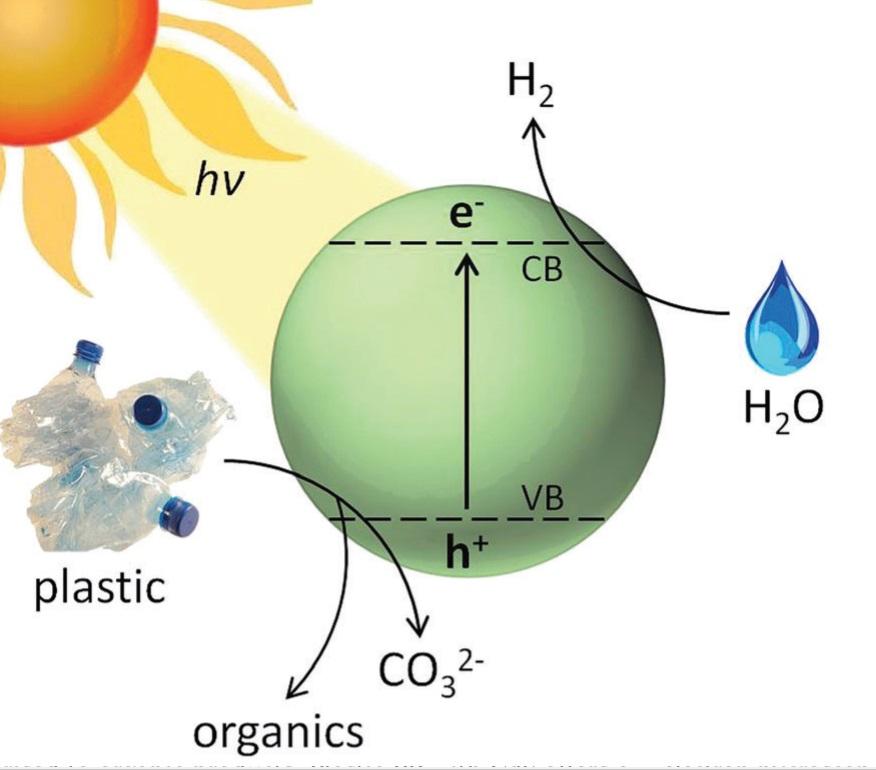
Picture this, non-recyclable waste reused to make use of its intrinsic energy, extending its life-cycle for that little bit longer in transforming it into hydrogen (H2), whilst alleviating plastic waste. Led by Professor Reisner Erwin, researchers from the Reisner Lab (Department of Chemistry, University of Cambridge) have demonstrated transformation of plastic into hydrogen by a process using sunlight.
The novel approach of photoreforming, enables the conversion of waste plastic into a renewable fuel source. It requires only visible light and a suitable metal-free photocatalyst at ambient temperature and pressure. The efficient process dropped cadmium sulfide quantum dots photocatalyst in alkaline aqueous solution on to three commonly used plastics: polylactic acid (PLA), polyethylene terephthalate (PET), polyurethane (PUR), including a PET water bottle. This was then irradiated with sunlight, reducing water from the solution to H2, while the plastic polymer simultaneously oxidised to small organic molecules.
The research is further enhanced by its applicability to use on plastics contaminated with food or oil. Recycling plastics to produce viable new plastics requires clean, pure materials, which eliminates unclean plastic waste. It validates real-world applicability of the system by converting a PET water bottle into H2. This is the first demonstration of visible light-driven, noble metal-free photoreforming of plastic.
The University of Cambridge’s research team includes Taylor Uekert from the Nanoscience and Nanotechnology NanoDTC.
Click here for her short video explaining the process.
Click here for the full article and image credit: Chemistry World

