Researchers have made a tiny camera, held together with ‘molecular glue’ that allows them to observe chemical reactions in real time.
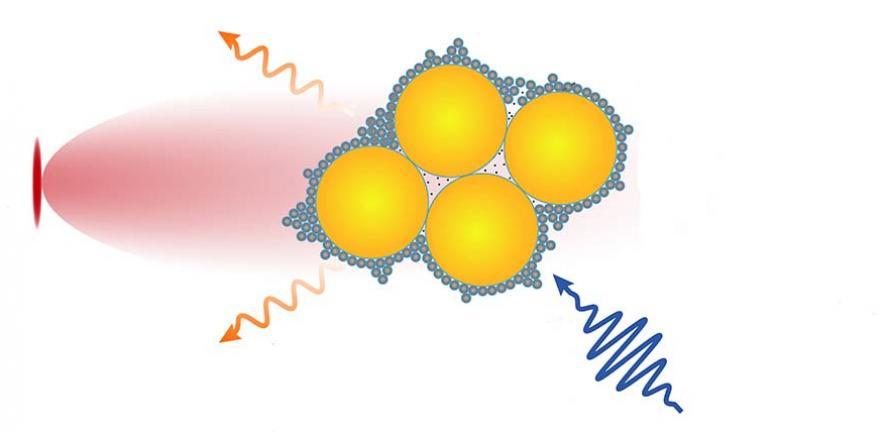
The device, made by a team from the University of Cambridge, combines tiny semiconductor nanocrystals called quantum dots and gold nanoparticles using molecular glue called cucurbituril (CB). When added to water with the molecule to be studied, the components self-assemble in seconds into a stable, powerful tool that allows the real-time monitoring of chemical reactions.
The camera harvests light within the semiconductors, inducing electron transfer processes like those that occur in photosynthesis, which can be monitored using incorporated gold nanoparticle sensors and spectroscopic techniques. They were able to use the camera to observe chemical species which had been previously theorised but not directly observed.
The platform could be used to study a wide range of molecules for a variety of potential applications, such as the improvement of photocatalysis and photovoltaics for renewable energy.
This platform is a really big toolbox – it opens up lots of new possibilities for imaging chemical reactionsKamil Sokołowski, Yusuf Hamied Department of Chemistry
The new method that Oren Scherman and his colleagues from Cambridge’s Cavendish Laboratory and University College London developed uses cucurbituril – a molecular glue which interacts strongly with both semiconductor quantum dots and gold nanoparticles. The researchers used small semiconductor nanocrystals to control the assembly of larger nanoparticles through a process they coined interfacial self-limiting aggregation. The process leads to permeable and stable hybrid materials that interact with light. The camera was used to observe photocatalysis and track light-induced electron transfer.
Researchers from the Scherman lab are currently working to further develop these hybrids towards artificial photosynthetic systems and (photo)catalysis where electron-transfer processes can be observed directly in real time. The team is also looking at mechanisms of carbon-carbon bond formation as well as electrode interfaces for battery applications.
Read the full University of Cambridge article here.
Click here for the Nature Nanotechnology (2021) publication.
Image: Nano camera. Credit: Scherman Group

