Researchers from the Catalysis and Process Integration group (Department of Chemical engineering and Biotechnology), led by Dr Laura Torrente, have developed a new way to produce ammonia. The new system redefines the century old Haber-Bosch process within an energy landscape shifting to renewables, where ‘green ammonia’ will have a role as a long-term energy vector.
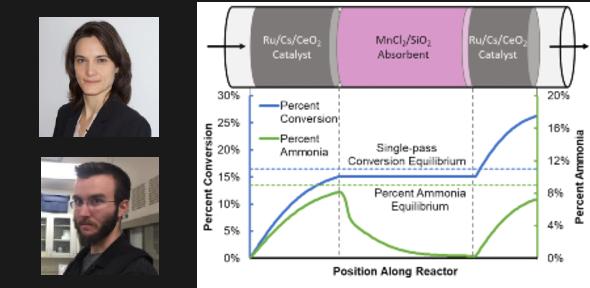
The research, published in Advanced Energy Materials, provides the proof-of-concept for a process tailored to be powered solely by intermittent and geographically isolated renewable energy, by combining ammonia synthesis and separation in a single vessel – leading to low-capital and fast response.
Industrial ammonia production is responsible for feeding 50% of people on Earth through fertilisers, but is also responsible for over 2% of global CO2 emissions. The Haber-Bosch process, that produces ammonia by combining nitrogen and hydrogen, was developed in the early 20th century. It is highly optimised and integrated but relies on the use of fossil fuels as both the hydrogen and energy source, producing what is called ‘brown ammonia’.
By shifting to renewable energy, with hydrogen derived from water, and nitrogen from air, the optimisation constraints change. enewable energy is isolated and intermittent, not centralised and continuous as required by the conventional Haber-Bosch process. Therefore, a new process is required for renewably-derived ammonia – known as ‘green ammonia’. By combining ammonia synthesis and separation in a single vessel, the need for a large recycle loop and heat exchange between the conventional reaction (run at temperatures above 400°C) and separation (condensation that requires temperatures below 0°C) is removed, leading to a low-capital and agile process.
We’re not trying to replace the conventional ammonia production on the same terms. We’re looking to distributed units that are lower capital, more agile and fitting for renewable energy."
Collin Smith, Catalysis and Process Integration, Department of Chemical Engineering and Biotechnology
Click here for the Department of Chemical Engineering and Biotechnology, University of Cambridge article.
"Exceeding Single‐Pass Equilibrium with Integrated Absorption Separation for Ammonia Synthesis Using Renewable Energy—Redefining the Haber‐Bosch Loop", Advanced Energy Materials (2021). DOI: 10.1002/aenm.202003845

